Metal components for drug delivery devices
Devices
Harm Reduction
The Complete Guide to Medical Device Component Projects
Metal Components for Drug Delivery Devices
Creating components with the exacting standards required for medical applications is testament to the engineering and craftsmanship that is a hallmark of Clamason Industries. Every component, no matter how small, plays a critical role in the overall functionality and safety of drug delivery. The integration of cutting-edge technology with traditional engineering expertise ensures these components meet the highest standards of precision reliability and safety. At the heart of this lies a deep understanding of the materials and processes involved. Advanced metal stamping and pressing techniques, combined with rigorous quality control measures, ensure each component not only meets but exceeds industry standards in precision.
Commitment to Innovation in Drug Delivery Devices
Innovation is the driving force behind the development of new and improved drug delivery devices. The demand for even more efficient, user-friendly, and safe devices is relentless, and meeting this demand requires a real commitment to technological advancement. This focus ensures the latest advancements in materials science, manufacturing technology, and design are incorporated into the development of metal components for drug delivery devices. This ethos is reflected in the investment in research and development. By staying at the forefront of technological advancements, it is possible to provide solutions that meet the evolving needs of the medical industry.
Medical Expertise and Professionalism
The production of metal components for drug delivery devices requires a blend of skill and expertise. From initial design through to final production, each step of the process is planned and executed to ensure the highest quality outcomes. This expertise encompasses a wide range of areas, including materials science, mechanical engineering, and quality control. One of the key aspects of this expertise is the ability to work closely with clients to understand their specific needs and requirements. By collaborating with medical device manufacturers, it is possible to develop customised solutions that are tailored to the challenges of each project.
In addition to design and manufacturing expertise, there is a strong emphasis on quality control. Rigorous testing and inspection procedures are in place at every stage of the production process to ensure each component meets the highest possible standards of quality and reliability. This attention to detail is especially important in the medical industry, where even the smallest defect can be the difference between life and death.
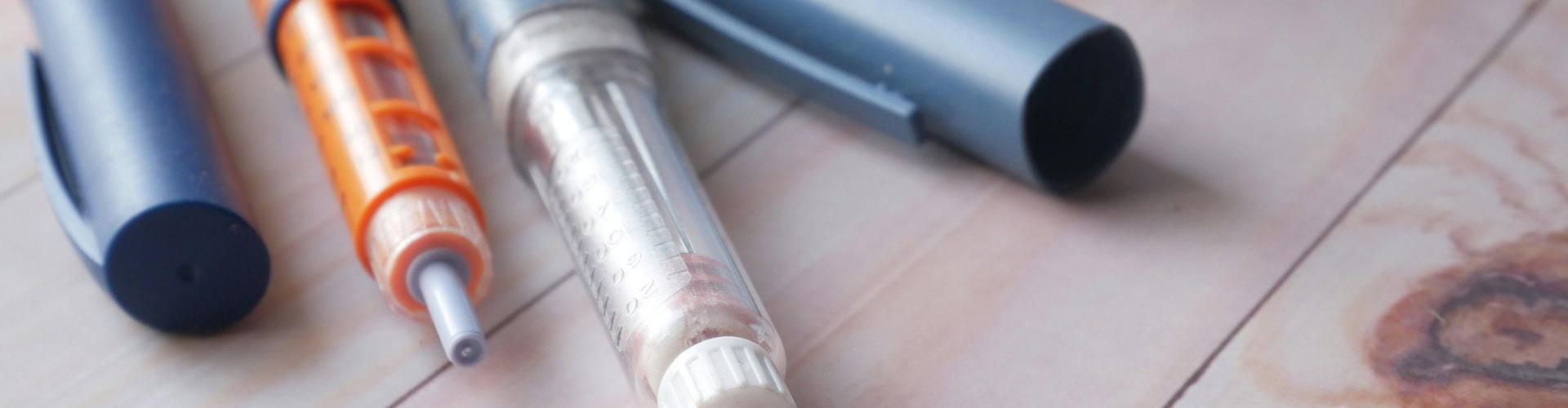
Sustainable Practices and Ethical Manufacturing
Our commitment to sustainable practices is evident in every aspect of the production process, from the sourcing of materials to the implementation of energy-efficient manufacturing techniques. By reducing waste and reducing environmental impact, it is possible to produce high-quality components in a way that is both environmentally responsible and economically viable. Ethical manufacturing practices are also a key focus, making sure all employees work in safe and fair conditions. By doing this, it is possible to build strong, lasting relationships with clients and suppliers, based on trust and mutual respect.
The Role of Metal Stamping in Medical Technologies
Metal stamping has emerged as a cornerstone technology in the manufacturing of medical devices, particularly those used for drug delivery. This precision-based process involves the conversion of metal sheets into complex components through techniques such as blanking, piercing, bending, and coining. The intricate designs and high tolerance levels required in medical devices are achievable through metal stamping, making it an indispensable technique in this sector.
The medical industry demands components that are not only precise but also consistent in quality. Metal stamping excels in producing parts with exacting specifications, ensuring each piece meets stringent standards for safety and functionality. For drug delivery devices, components such as springs, housings, and connectors must fit together perfectly to ensure reliable operation. Metal stamping provides this high level of accuracy needed to create these intricate parts. Advanced machinery and computer-aided design (CAD) software enable the production of components with microscopic tolerances, ensuring uniformity across large production runs.
Advantages of Progressive Tooling and Stamping Simulation
Progressive tooling offers significant advantages over traditional compound tooling. It allows for the creation of complex geometries by forming different attributes into the part at various stages. This staged process enables higher complexity and speed in production. Maintenance is also easier with progressive tooling, as it allows focus on different stages rather than the entire tool, making breakdowns less disruptive and the tool preparation more efficient. Stamping simulation plays a critical role in the design and testing of medical components. By simulating the forces during the stamping process, it is possible to identify potential issues such as cracking due to excessive force. This simulation helps ensure the material forms as expected and guides the tool design process. Before designing the actual tool, simulations of all stages of the progressive tool are conducted to prevent adverse effects from the various forces applied during the stamping process.
At Clamason Industries, the ability to manufacture components to a very accurate tolerance is testament to our engineering heritage. We can produce millions of parts consistently to those tolerances, ensuring each piece meets stringent standards for safety and functionality. To maintain these high standards, we employ a control method known as process capability, a statistical approach used to ensure all key characteristics and dimensions of the parts remain not only within specifications but also stable, producing consistent results. This method helps us monitor and control our manufacturing processes effectively. Our high-performance laboratory is equipped with advanced measuring devices, both visual and tactile, which enable us to measure key characteristics accurately and ensure stable performance.
For our medical tools, which are produced in high volumes, we implement planned maintenance on the tooling to prevent deterioration that could lead to defects. We also conduct reactive maintenance based on ongoing measurements throughout the manufacturing process. If we detect a dimension veering out of spec, we can pinpoint the specific part of the tool that needs adjustment to bring the part back into spec. This continuous monitoring and feedback loop into our maintenance process to ensure the highest quality and reliability in our components. By integrating these advanced methodologies and rigorous quality control measures, we ensure each component meets the highest standards of precision and reliability.
Reliability
Medical devices, particularly those involved in drug delivery, must withstand rigorous use and environmental conditions without compromising performance. Metals such as stainless steel, titanium, and aluminium offer exceptional strength and resistance to corrosion, making them ideal for medical applications. The stamping process enhances the intrinsic properties of these metals, producing components that can endure repetitive use and exposure to chemicals and sterilisation processes

Cost-Effective Precision
Metal stamping is not only precise but also efficient, allowing for mass production of components at a lower cost compared to other manufacturing methods. The ability to produce large quantities of parts quickly and accurately reduces production time and cost. Metal stamping’s high repeatability ensures that each component produced is identical, maintaining the strict quality standards required in the medical industry.
Flexibility
The versatility of metal stamping allows for a wide range of applications in medical device manufacturing. Whether it’s creating tiny, intricate components for an insulin pump or larger, robust parts for a surgical instrument, metal stamping can accommodate a variety of design requirements. This is crucial in the medical field, where devices are often tailored to meet specific patient needs. Advanced stamping techniques, such as progressive die stamping and deep drawing, enable the creation of complex geometries and thin-walled structures that are difficult to achieve through other manufacturing processes. This allows for innovative designs that enhance the functionality and usability of medical devices.
Quality Counts
Safety is paramount in the medical device industry. The precision and reliability offered by metal stamping contributes to the overall safety of medical devices. By producing components that meet exact spec, manufacturers can ensure their devices operate exactly as intended and comply with industry regulations. Quality control is a big part of the process. Each component undergoes thorough inspection and testing to verify its conformity to design specifications and performance criteria. Metal stamping supports this by enabling the development of versatile drug delivery devices. From wearable injectors providing continuous medication administration to autoinjectors that ensure that precise dose of medicine, metal components are just the job. This ability to produce complex, high-precision parts allows engineers to push the boundaries of what is possible in drug delivery.

Overview of Products and Solutions
Introduction to Key Products
Metal components are essential in ensuring precision, durability, and safety in devices such as insulin pens, dry powder inhalers, and RNS removers. Each product represents a significant advance in medical tech, aimed at improving patient outcomes and providing efficient, user-friendly solutions.
Insulin Pens
This invention has revolutionised the management of diabetes, offering patients a convenient and precise method of insulin administration. The metal components within these devices are critical to their performance. High-precision metal parts such as needles, springs, and dose selectors ensure easy, smooth and accurate operation. The manufacturing process for these components involves advanced metal stamping techniques, allowing for the production of small, intricate parts with high tolerances. These components must meet stringent quality standards to ensure that each dose of insulin is delivered accurately and consistently. The use of durable materials like stainless steel ensures the pens are robust and capable of withstanding repeated use without wearing out. In this instance the technology involved really can change people’s lives for the better.
Dry Powder Inhalers
Dry powder inhalers (DPIs) are another category of drug delivery device for patients with respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD). These devices rely heavily on the precise engineering of their metal components to function effectively. Key parts include springs, actuators, and dispersion plates. These components work together to ensure the correct dose of medication is delivered to the patient’s lungs. The springs and actuators are responsible for the device’s mechanical action, releasing the medication when the patient inhales. The dispersion plates ensure the powder is properly atomised, enhancing delivery and absorption.
Quality and Innovation
The production of metal components for drug delivery devices demands a commitment to quality and innovation. Each component must undergo rigorous testing and quality control measures to ensure it meets the highest standards of precision and reliability. Advanced manufacturing techniques, such as computer-aided design (CAD) and computer-aided manufacturing (CAM), are used to create components with exceptional accuracy and consistency. Ideas play a vital role in the continuous improvement of these components. Research and development efforts focus on exploring new materials, enhancing manufacturing processes, and developing more efficient designs. Through precision engineering, commitment to quality, and continuous innovation, these metal components help enhance patient care and improve the overall effectiveness of drug delivery systems.
Accreditations and Quality Assurance
Understanding ISO 13485 Accreditation
Adherence to stringent quality standards in medicine is non-negotiable. ISO 13485 accreditation represents a critical benchmark for quality management systems in this industry. This internationally recognised standard is specifically designed to ensure that organisations involved in the design, production, and servicing of medical devices consistently meet regulatory requirements and customer expectations. ISO 13485 sets out the criteria for a comprehensive quality management system (QMS) that governs every aspect of the manufacturing process. Achieving this accreditation signifies a commitment to maintaining the highest standards of quality, safety, and efficacy in medical device production. The standard focuses on risk management, traceability, and stringent process controls, ensuring that every product meets the necessary safety and performance standards.

Commitment to Quality
The pursuit of ISO 13485 accreditation reflects a dedication to quality and continuous improvement. This commitment is embedded in every facet of the manufacturing process, from initial design and development to final production and delivery. Key elements include robust documentation practices, comprehensive risk management strategies, and a culture of quality that permeates the entire organisation. Detailed records are maintained at every stage of production, ensuring complete traceability and accountability.
Quality Control Process
Quality control begins with the selection of raw materials. Only high-grade metals that meet the necessary specifications are used in the manufacturing process. These materials undergo rigorous testing to verify their composition, strength, and durability. Once the raw materials are approved, they are subjected to precise machining and fabrication processes, with each step closely monitored to ensure adherence to quality standards. Throughout the process, components are inspected at various points to identify any deviation from the required specifications.
Advanced inspection technologies such as coordinate measuring machines (CMMs) and optical comparators are used to perform detailed measurements and assessments. These tools provide accurate, repeatable results, ensuring each component meets the highest standards of precision and quality.
The medical version of PPAP (Production Part Approval Process) is called GMP (Good Manufacturing Practice) validation. Unlike PPAP, which has a set procedure for validation documents, GMP is risk-based. It involves conducting a risk assessment to validate high-risk parts of the process. Key areas identified as high risk undergo rigorous validation, which may include run-at-rate trials. During these trials, the press tool is run for at least two hours to ensure parts produced are consistent and the process is sustainable. This means there should be no downtime due to breakdowns, and key aspects such as material changes and coil replacements are efficient and do not affect component quality.
The results of these trials are recorded in validation documents and approved by the customer. This approach goes beyond just ensuring component quality, focusing on the entire process’s capability and reliability.
Risk Management and Mitigation
Risk management is a cornerstone of ISO 13485, emphasising the importance of identifying and addressing potential risks throughout the product lifecycle. A comprehensive risk management strategy involves the systematic identification, assessment, and mitigation of risks associated with the design, production, and use of medical devices. This proactive approach to risk management helps to prevent defects and ensure the safety and reliability of medical devices.
Continuous Improvement
This is a fundamental principle of ISO 13485, driving organisations to constantly evaluate and enhance their processes and products. This commitment to continuous improvement ensures that manufacturers stay at the forefront of technological advancements and maintain their competitive edge in the medical device industry. Regular internal audits and management reviews are conducted to assess the effectiveness of the quality management system and identify opportunities for improvement. Feedback from customers and other stakeholders is also actively sought and used to inform improvements in product design, manufacturing processes, and customer service.
Customer safety satisfaction is the goal at Clamason Industries. By consistently delivering high-quality products that meet or exceed customer expectations, manufacturers build trust and establish long-term relationships with their clients. The rigorous quality controls and continuous improvement efforts required by ISO 13485 help to ensure that customers receive reliable, safe, and effective medical devices. ISO 13485 accreditation and robust quality assurance processes are essential for the successful production of metal components for drug delivery devices.
Case Studies Transforming Insulin Delivery with Precision Components
A recent collaboration between Clamason Industries and a leading pharmaceutical company exemplifies how high-quality metal components can enhance the performance of life saving insulin pens.
The challenge was to develop a series of metal parts that would ensure the insulin pens operated flawlessly, providing precise dosages with every use. The components required included fine needles, springs, and dose selectors, all of which had to meet stringent specifications for accuracy and durability.
Advanced metal stamping techniques were employed to produce these intricate parts with the necessary precision. The result was a highly reliable insulin pen that performed consistently across numerous tests. This successful implementation not only improved the product but also reinforced the reputation of the pharmaceutical company as a provider of dependable and user-friendly diabetes management solutions. Patients benefited from the enhanced accuracy and ease of use, leading to better adherence to insulin therapy and improved health outcomes.

Enhancing Respiratory Therapy with Advanced Inhaler Components
Dry powder inhalers (DPIs) are critical for patients with respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD). A notable project involved partnering with a top-tier medical device manufacturer to improve the performance and reliability of their DPIs. The objective was to create components that would ensure the inhaler delivered the correct dosage of medication effectively and efficiently. The metal components included precision-engineered springs, actuators, and dispersion plates.
Using cutting-edge metal stamping and fabrication techniques, the components were produced with high accuracy and consistency. The collaboration resulted in an inhaler that met and exceeded industry standards for drug delivery performance. This not only enhanced the device’s market competitiveness but also provided patients with a more reliable and effective tool for managing their respiratory conditions. The project underscored the critical role of collaboration and precision engineering in developing medical devices that significantly improve patient health and well-being.

Custom Solutions for Unique Challenges
A key strength of Clamason Industries lies in the ability to provide customised solutions for unique challenges in the medical device industry. One such example involved working with a company developing a novel drug delivery system for targeted cancer therapy.
The collaboration involved close communication and iterative design processes to develop components that met the specific requirements of the drug delivery system. Advanced testing and quality control measures ensured each component performed reliably under the conditions of cancer therapy. The successful development and implementation of these components enabled the launch of an innovative drug delivery system that offered new hope for cancer patients.
This case study highlights the importance of bespoke, collaborative engineering solutions in addressing the unique challenges of medical device development and underscores the commitment to advancing healthcare through precision engineering. These success stories illustrate the significant impact that precision-engineered metal components have on the development and performance of drug delivery devices. Through collaboration, innovation, and a commitment to quality, these projects have led to the creation of reliable, effective, and life-enhancing medical devices. Whether improving existing technologies or developing new solutions, our focus remains on delivering the highest standards of quality and performance to advance healthcare and improve patient outcomes.
Contact Clamason Industries now and let us help with your latest medical component needs.
Related articles and insights
Metal Cleaning & Contract Services Infographic

Thin Gauge Stainless Steel Pressing: Is It Right For Your Project?
Thin Gauge Stainless Steel Pressing: Is It Right For Your Project? Manufacturers working in the medical, automotive, electronics and consumer goods sectors use thin gauge stainless steel primarily because it provides strength along with formability and corrosion...
How Does High Precision Metal Pressing Improve Accuracy?
In industrial sectors where even the smallest deviation in precision can jeopardise safety, functionality, or performance, manufacturing accuracy is vital. This ability to maintain quality and consistency is especially relevant in...
Ensuring Regulatory Compliance in Metal Stamping for Medical Devices
Stamping Regulations Metal stamping, a key process in the production of medical devices, must adhere to stringent regulatory standards to meet industry requirements. Here we will examine the importance of regulatory compliance in metal stamping for medical devices,...
